The electromagnetic (EM) spectrum is the range of all types of EM radiation. Radiation is energy that travels and spreads out as it goes – the visible light that comes from a lamp in your house and the radio waves that come from a radio station are two types of electromagnetic radiation. The other types of EM radiation that make up the electromagnetic spectrum are microwaves, infrared light, ultraviolet light, X-rays and gamma-rays.
The types of electromagnetic radiation are broadly classified into the following classes
EM waves in everyday life.
Radio: Your radio captures radio waves emitted by radio stations, bringing your favorite tunes. Radio waves are also emitted by stars and gases in space.
Microwave: Microwave radiation will cook your popcorn in just a few minutes, but is also used by astronomers to learn about the structure of nearby galaxies.
Infrared: Night vision goggles pick up the infrared light emitted by our skin and objects with heat. In space, infrared light helps us map the dust between stars.
Visible: Our eyes detect visible light. Fireflies, light bulbs, and stars all emit visible light.
Ultraviolet: Ultraviolet radiation is emitted by the Sun and are the reason skin tans and burns. "Hot" objects in space emit UV radiation as well.
X-ray: A dentist uses X-rays to image your teeth, and airport security uses them to see through your bag. Hot gases in the Universe also emit X-rays.
Gamma ray: Doctors use gamma-ray imaging to see inside your body. The biggest gamma-ray generator of all is the Universe.
Radio waves can be made to carry information by varying a combination of the amplitude, frequency, and phase of the wave within a frequency band. When EM radiation impinges upon a conductor, it couples to the conductor, travels along it, and induces an electric current on the surface of that conductor by exciting the electrons of the conducting material. This effect (the skin effect) is used in antennas.
The super-high frequency (SHF) and extremely high frequency (EHF) of microwaves come after radio waves. Microwaves are waves that are typically short enough to employ tubular metal waveguides of reasonable diameter. Microwave energy is produced with klystron and magnetron tubes, and with solid state diodes such as Gunn and IMPATT devices. Microwaves are absorbed by molecules that have a dipole moment in liquids. In a microwave oven, this effect is used to heat food. Low-intensity microwave radiation is used in Wi-Fi, although this is at intensity levels unable to cause thermal heating.
Volumetric heating, as used by microwave ovens, transfers energy through the material electromagnetically, not as a thermal heat flux. The benefit of this is a more uniform heating and reduced heating time; microwaves can heat material in less than 1% of the time of conventional heating methods.
When active, the average microwave oven is powerful enough to cause interference at close range with poorly shielded electromagnetic fields such as those found in mobile medical devices and cheap consumer electronics.
Electromagnetic radiation with a wavelength between 380 nm and 760 nm (400-790 terahertz) is detected by the human eye and perceived as visible light. Other wavelengths, especially near infrared (longer than 760 nm) and ultraviolet (shorter than 380 nm) are also sometimes referred to as light, especially when the visibility to humans is not relevant. White light is a combination of lights of different wavelengths in the visible spectrum. Passing white light through a prism splits it up into the several colors of light observed in the visible spectrum between 400 nm and 780 nm.
If radiation having a frequency in the visible region of the EM spectrum reflects off an object, say, a bowl of fruit, and then strikes our eyes, this results in our visual perception of the scene. Our brain's visual system processes the multitude of reflected frequencies into different shades and hues, and through this insufficiently-understood psychophysical phenomenon, most people perceive a bowl of fruit.
At most wavelengths, however, the information carried by electromagnetic radiation is not directly detected by human senses. Natural sources produce EM radiation across the spectrum, and technology can also manipulate a broad range of wavelengths. Optical fiber transmits light that, although not necessarily in the visible part of the spectrum (it is usually infrared), can carry information. The modulation is similar to that used with radio waves.
Next in frequency comes ultraviolet (UV). The wavelength of UV rays is shorter than the violet end of the visible spectrum but longer than the X-ray.
UV in the very shortest range (next to X-rays) is capable even of ionizing atoms (see photoelectric effect), greatly changing their physical behavior.
At the middle range of UV, UV rays cannot ionize but can break chemical bonds, making molecules to be unusually reactive. Sunburn, for example, is caused by the disruptive effects of middle range UV radiation on skin cells, which is the main cause of skin cancer. UV rays in the middle range can irreparably damage the complex DNA molecules in the cells producing thymine dimers making it a very potent mutagen.
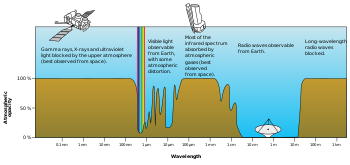
The Sun emits significant UV radiation (about 10% of its total power), including extremely short wavelength UV that could potentially destroy most life on land (ocean water would provide some protection for life there). However, most of the Sun's most-damaging UV wavelengths are absorbed by the atmosphere's oxygen, nitrogen, and ozone layer before they reach the surface. The higher ranges of UV (vacuum UV) are absorbed by nitrogen and, at longer wavelengths, by simple diatomic oxygen in the air. Most of the UV in this mid-range is blocked by the ozone layer, which absorbs strongly in the important 200–315 nm range, the lower part of which is too long to be absorbed by ordinary dioxygen in air. The range between 315 nm and visible light (called UV-A) is not blocked well by the atmosphere, but does not cause sunburn and does less biological damage. However, it is not harmless and does cause oxygen radicals, mutation and skin damage. See ultraviolet for more information.
Source:
http://imagine.gsfc.nasa.gov/docs/science/know_l1/emspectrum.html
http://en.wikipedia.org/wiki/Electromagnetic_spectrum
The types of electromagnetic radiation are broadly classified into the following classes
- Gamma radiation
- X-ray radiation
- Ultraviolet radiation
- Visible radiation
- Infrared radiation
- Terahertz radiation
- Microwave radiation
- Radio waves
EM waves in everyday life.
Radio: Your radio captures radio waves emitted by radio stations, bringing your favorite tunes. Radio waves are also emitted by stars and gases in space.
Microwave: Microwave radiation will cook your popcorn in just a few minutes, but is also used by astronomers to learn about the structure of nearby galaxies.
Infrared: Night vision goggles pick up the infrared light emitted by our skin and objects with heat. In space, infrared light helps us map the dust between stars.
Visible: Our eyes detect visible light. Fireflies, light bulbs, and stars all emit visible light.
Ultraviolet: Ultraviolet radiation is emitted by the Sun and are the reason skin tans and burns. "Hot" objects in space emit UV radiation as well.
X-ray: A dentist uses X-rays to image your teeth, and airport security uses them to see through your bag. Hot gases in the Universe also emit X-rays.
Gamma ray: Doctors use gamma-ray imaging to see inside your body. The biggest gamma-ray generator of all is the Universe.
Radio frequency
Radio waves generally are utilized by antennas of appropriate size (according to the principle of resonance), with wavelengths ranging from hundreds of meters to about one millimeter. They are used for transmission of data, via modulation. Television, mobile phones, wireless networking, and amateur radio all use radio waves. The use of the radio spectrum is regulated by many governments through frequency allocation.Radio waves can be made to carry information by varying a combination of the amplitude, frequency, and phase of the wave within a frequency band. When EM radiation impinges upon a conductor, it couples to the conductor, travels along it, and induces an electric current on the surface of that conductor by exciting the electrons of the conducting material. This effect (the skin effect) is used in antennas.
Microwaves
Main article: Microwaves
Volumetric heating, as used by microwave ovens, transfers energy through the material electromagnetically, not as a thermal heat flux. The benefit of this is a more uniform heating and reduced heating time; microwaves can heat material in less than 1% of the time of conventional heating methods.
When active, the average microwave oven is powerful enough to cause interference at close range with poorly shielded electromagnetic fields such as those found in mobile medical devices and cheap consumer electronics.
Terahertz radiation
Main article: Terahertz radiation
Terahertz radiation is a region of the spectrum between far infrared
and microwaves. Until recently, the range was rarely studied and few
sources existed for microwave energy at the high end of the band
(sub-millimeter waves or so-called terahertz waves),
but applications such as imaging and communications are now appearing.
Scientists are also looking to apply terahertz technology in the armed
forces, where high-frequency waves might be directed at enemy troops to
incapacitate their electronic equipment.[17]Infrared radiation
Main article: Infrared radiation
The infrared
part of the electromagnetic spectrum covers the range from roughly
300 GHz (1 mm) to 400 THz (750 nm). It can be divided into three parts:[3]- Far-infrared, from 300 GHz (1 mm) to 30 THz (10 μm). The lower part of this range may also be called microwaves. This radiation is typically absorbed by so-called rotational modes in gas-phase molecules, by molecular motions in liquids, and by phonons in solids. The water in Earth's atmosphere absorbs so strongly in this range that it renders the atmosphere in effect opaque. However, there are certain wavelength ranges ("windows") within the opaque range that allow partial transmission, and can be used for astronomy. The wavelength range from approximately 200 μm up to a few mm is often referred to as "sub-millimeter" in astronomy, reserving far infrared for wavelengths below 200 μm.
- Mid-infrared, from 30 to 120 THz (10 to 2.5 μm). Hot objects (black-body radiators) can radiate strongly in this range, and human skin at normal body temperature radiates strongly at the lower end of this region. This radiation is absorbed by molecular vibrations, where the different atoms in a molecule vibrate around their equilibrium positions. This range is sometimes called the fingerprint region, since the mid-infrared absorption spectrum of a compound is very specific for that compound.
- Near-infrared, from 120 to 400 THz (2,500 to 750 nm). Physical processes that are relevant for this range are similar to those for visible light. The highest frequences in this region can be detected directly by some types of photographic film, and by many types of solid state image sensors for infrared photography and videography.
Visible radiation (light)
Main article: Visible spectrum
Above infrared in frequency comes visible light. The Sun
emits its peak power in the visible region, although integrating the
entire emission power spectrum through all wavelengths shows that the
Sun emits slightly more infrared than visible light.[18] By definition, visible light is the part of the EM spectrum to which the human eye
is the most sensitive. Visible light (and near-infrared light) is
typically absorbed and emitted by electrons in molecules and atoms that
move from one energy level to another. This action allows the chemical
mechanisms that underly human vision and plant photosynthesis. The light
which excites the human visual system is a very small portion of the electromagnetic spectrum. A rainbow
shows the optical (visible) part of the electromagnetic spectrum;
infrared (if you could see it) would be located just beyond the red side
of the rainbow with ultraviolet appearing just beyond the violet end.Electromagnetic radiation with a wavelength between 380 nm and 760 nm (400-790 terahertz) is detected by the human eye and perceived as visible light. Other wavelengths, especially near infrared (longer than 760 nm) and ultraviolet (shorter than 380 nm) are also sometimes referred to as light, especially when the visibility to humans is not relevant. White light is a combination of lights of different wavelengths in the visible spectrum. Passing white light through a prism splits it up into the several colors of light observed in the visible spectrum between 400 nm and 780 nm.
If radiation having a frequency in the visible region of the EM spectrum reflects off an object, say, a bowl of fruit, and then strikes our eyes, this results in our visual perception of the scene. Our brain's visual system processes the multitude of reflected frequencies into different shades and hues, and through this insufficiently-understood psychophysical phenomenon, most people perceive a bowl of fruit.
At most wavelengths, however, the information carried by electromagnetic radiation is not directly detected by human senses. Natural sources produce EM radiation across the spectrum, and technology can also manipulate a broad range of wavelengths. Optical fiber transmits light that, although not necessarily in the visible part of the spectrum (it is usually infrared), can carry information. The modulation is similar to that used with radio waves.
Ultraviolet light
Main article: Ultraviolet
UV in the very shortest range (next to X-rays) is capable even of ionizing atoms (see photoelectric effect), greatly changing their physical behavior.
At the middle range of UV, UV rays cannot ionize but can break chemical bonds, making molecules to be unusually reactive. Sunburn, for example, is caused by the disruptive effects of middle range UV radiation on skin cells, which is the main cause of skin cancer. UV rays in the middle range can irreparably damage the complex DNA molecules in the cells producing thymine dimers making it a very potent mutagen.
The Sun emits significant UV radiation (about 10% of its total power), including extremely short wavelength UV that could potentially destroy most life on land (ocean water would provide some protection for life there). However, most of the Sun's most-damaging UV wavelengths are absorbed by the atmosphere's oxygen, nitrogen, and ozone layer before they reach the surface. The higher ranges of UV (vacuum UV) are absorbed by nitrogen and, at longer wavelengths, by simple diatomic oxygen in the air. Most of the UV in this mid-range is blocked by the ozone layer, which absorbs strongly in the important 200–315 nm range, the lower part of which is too long to be absorbed by ordinary dioxygen in air. The range between 315 nm and visible light (called UV-A) is not blocked well by the atmosphere, but does not cause sunburn and does less biological damage. However, it is not harmless and does cause oxygen radicals, mutation and skin damage. See ultraviolet for more information.
X-rays
Main article: X-rays
After UV come X-rays,
which, like the upper ranges of UV are also ionizing. However, due to
their higher energies, X-rays can also interact with matter by means of
the Compton effect.
Hard X-rays have shorter wavelengths than soft X-rays. As they can pass
through most substances with some absorption, X-rays can be used to
'see through' objects with thicknesses less than equivalent to a few
meters of water. The most notable use in this category being diagnostic
X-ray images in medicine (a process known as radiography). X-rays are useful as probes in high-energy physics. In astronomy Neutron stars and accretion disks around black holes
emit X-rays, which enable us to study them. X-rays are also emitted by
stars and are strongly emitted by some types of nebulae. However, X-ray telescopes must be placed outside the Earth's atmosphere to see astronomical X-rays, since the atmosphere of Earth
has a density equivalent to about 10 meters of water, which is
sufficient to block almost all astronomical X-rays (and also
astronomical gamma rays—see below).Gamma rays
Main article: Gamma rays
After hard X-rays come gamma rays, which were discovered by Paul Villard in 1900. These are the most energetic photons, having no defined lower limit to their wavelength. In astronomy
they are valuable for studying high-energy objects or regions, however
like with X-rays this can only be done with telescopes outside the
Earth's atmosphere. Gamma rays are useful to physicists thanks to their
penetrative ability and their production from a number of radioisotopes. Gamma rays are also used for the irradiation of food and seed for sterilization, and in medicine they are occasionally used in radiation cancer therapy. More commonly, gamma rays are used for diagnostic imaging in nuclear medicine, with an example being PET scans. The wavelength of gamma rays can be measured with high accuracy by means of Compton scattering.Source:
http://imagine.gsfc.nasa.gov/docs/science/know_l1/emspectrum.html
http://en.wikipedia.org/wiki/Electromagnetic_spectrum


No comments:
Post a Comment